Bugula neritina and Neosiphonia harveyi
One of the issues facing a biologist out in the field when trying to identify marine species is matching the photographs in guides with what is seen with fresh material. Guides do not give examples of all the different morphological or color variants. Nor do they show the appearance of species at different ages (sizes) or stages of the life cycle. In my experience, an unfamiliar species may require a few occasions to sort out the distinctions. This was the case for me with Bugula neritina. It has an algae-like, bushy growth pattern and is sometimes not abundant enough to form an immediate identification. Using a 30x or 40x hand lens in the field or bringing samples back to the lab for microscopic confirmation have been a big help. In due time, the identification process gets refined.
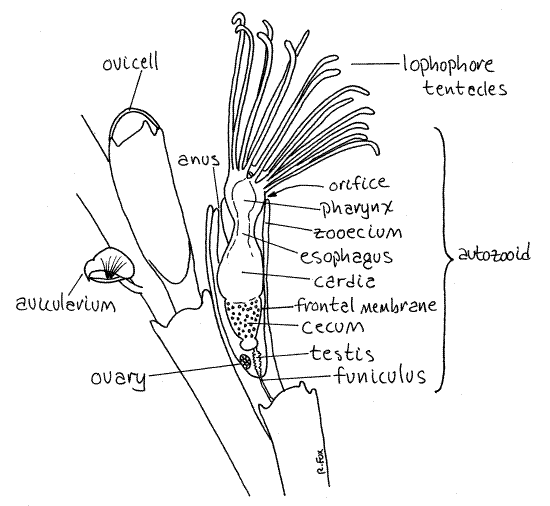
Among the red algaes, Neosiphonia harveyi is one species that can cause confusion when attempting to identify Bugula neritina, especially when the specimens are young (only a few cm in height) and neither of them has developed reproductive structures. Side by side in a collection tray, the macroscopic differences can be easily discerned. Under a microscope, however, the structural differences are crystal clear. Bugula has serrated edges and alternating biserial growth. It is an animal with moving, feeding lophophores that contract down into their enclosures at the slightest disturbance and then slowly reappear to resume feeding (very entertaining). Neosiphonia is a plant that has smooth, striped, non-moving branches (thalli) with pointed tips. Each of the two species has unique, visible reproductive structures. Bugula has white ovicells along mature branches, whereas Neosiphonia has pod-like carposporangia, both of which can been seen with the naked eye or hand lens.
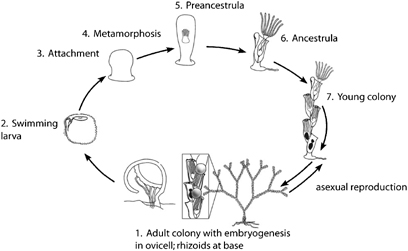
Neosiphonia harveyi, previously classified as Polysiphonia harveyi (Choi et al, 2001), is a multicellular red alga with two macroscopic phases to its life cycle: a haploid, reproductive structure that produces gametes and a diploid form that produces spores for asexual reproduction. The multicellullar branches in both stages of Polysiphonia and Neosiphonia species are composed of 5 primary cells in cross section, one central and 4 peripheral (see Algaebase link below). Both stages look the same, i.e. they are isomorphic, and in Neosiphonia, the male and female structures are produced on the same plant.
Bugula neritina and Neosiphonia harveyi with Similar Appearance and Size
Wine red specimens of Bugula neritina (left) and Neosiphonia harveyi (right) collected from MacMillan Wharf in Provincetown, September, 2012. Both the bryozoan and red alga branch with a similar bifurcating pattern, but branches of Bugula appear thicker and serrated. Neosiphonia has a filamentous appearance but is actually composed of multicellular thalli (red algal fronds). Neither specimen has conspicuous reproductive structures.
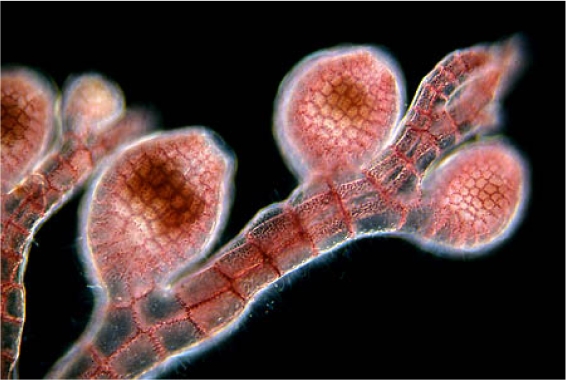
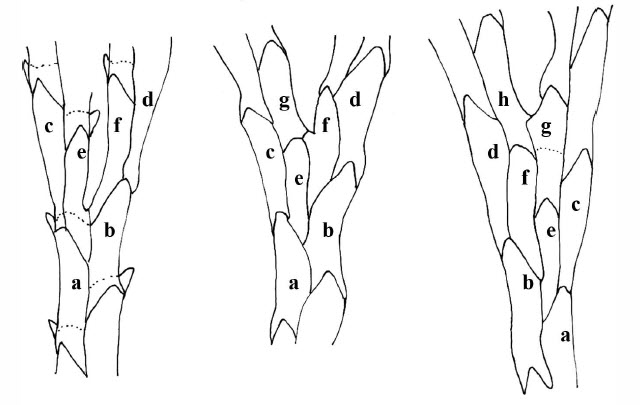
Microscopic View of Terminal Branches of Bugula neritina
Alternating zooids along the branch of Bugula with a cluster of feeding lophophores at the terminal end. Each branch is two rows of zooids wide. Serrated edges and alternating zooids can be with a hand lens. Lophophores can also be seen with a lens on resting colonies in a shallow dish. Stereozoom 4 x 10x objective.
Microscopic Views of Thalli of Neosiphonia harveyi
Specimen was collected from Provincetown, September, 2012. The segmented branches have red longitudinal and inter-segment bands. This structure can be seen with a 30x hand lens. Branch ends taper to a tip. Stereozoom 4.5 (upper) and 4.0 (lower) x 10x objective.
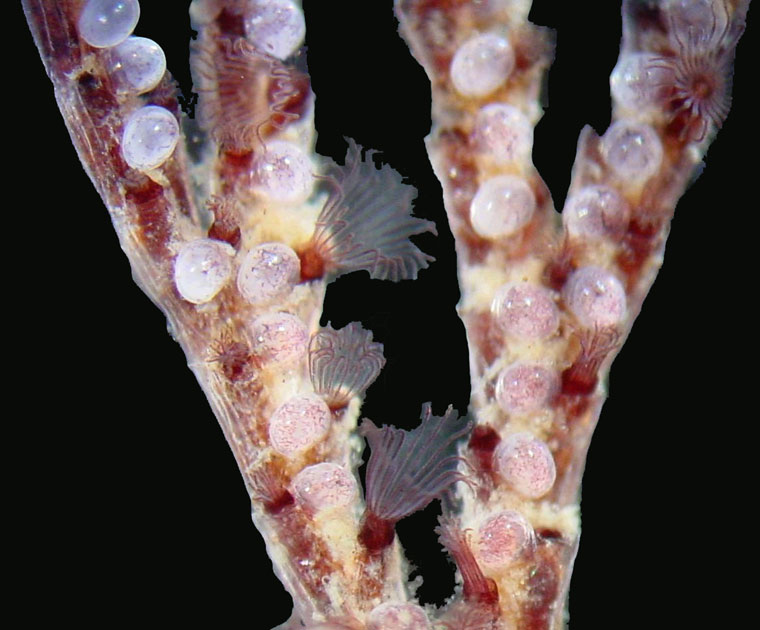
Neosiphonia harveyii Thalli with Carposporangia
Specimen was collected from Wellfleet, July, 2011. Carposporangia containing the newly fertilized (diploid) carposporophyte are located on short branches off the thallus. The carposporophyte produces carpospores which are released into the sea water.
Neosiphonia harveyii Growing as an Epiphyte on Grateloupia turuturu
Neosiphonia harveyi can be found growing as an epiphyte on several different algae. Here it is seen on the MIS invasive species Grateloupia turuturu, which is becoming more common in the Gulf of Maine, especially in late summer. From MacMillan Wharf, Provincetown, September 2012.
LINKS:
MarLIN: The Marine Life Information Network, Biodiversity & Conservation. Descriptions of major taxonomic groups.
The life cycle of red algae involves the alternation of three stages: the gametophyte, the carposporophyte, and the tetrasporophyte. Typical red algae have separate male and female gametophytes, but Neosiphonia has both on the same plant. The carposporophyte is microscopic and attached to the gametophyte.
WoRMS: World Registry of Marine Species. Neosiphonia harveyi (J.W.Bailey) M.-S. Kim, H.-G. Choi, M.D. Guiry & G.W. Saunders, 2001.
Algaebase. Neosiphonia harveyi (J.W. Bailey). Database of information on algae, especially marine algae.

Colorized cross section through a thallus showing cellular structure.
Plant Physiology Information Website by Ross E Koning: Kingdom Rhodophyta: Neosiphonia harveyi. The life cycle is described and well illustrated.
Carposporangia on haploid plants contain a microscopic diploid carposporophyte after fertilization.
Marevita (Sea Life) - Algues et Plantes Marines - Rhodophyta: Neosiphonia harveyi.
Tetrasporangia spiral along the diploid thallus. Carposporangia are produced on short stalks off the haploid thallus. (Photo credit: Andre Rio)
PUBLICATIONS:
Choi, H-G, M-S Kim, MD Guiry, and GW Saunders. Phylogenetic relationships of Polysiphonia (Rhodomelaceae, Rhodophyta) and its relatives based on anatomical and nuclear small-subunit rDNA sequence data. Canadian Journal of Botany 79: 1465-1476, 2001.
Morphological features of the A) thallus, B) rhizoid, C) carpogonium D) spermatangia, and E) tetraspores of i) Polysiphonia, iii) Neosiphonia, and ii) a multicentral relative.
Phylogenic relationships and reclassification of Polysiphonia harveyi as
Neosiphonia harveyi based on morphological criteria
Mathieson AC, JR Pederson, CD Neefus, CJ Dawes, and TL Bray. Multiple assessments of introduced seaweeds in the Northwest Atlantic. ICES Journal of Marine Science, 65: 730–741, 2008.